Ap Chem Equilibrium Frq



In this video Paul Andersen explains how equilibrium is achieved in a reversible reaction. When the rate of the forward reaction is equal to the rate of the reverse reaction the system is at equilibrium. Graphical analysis of equilibrium is included along with a walkthrough of several calculations.
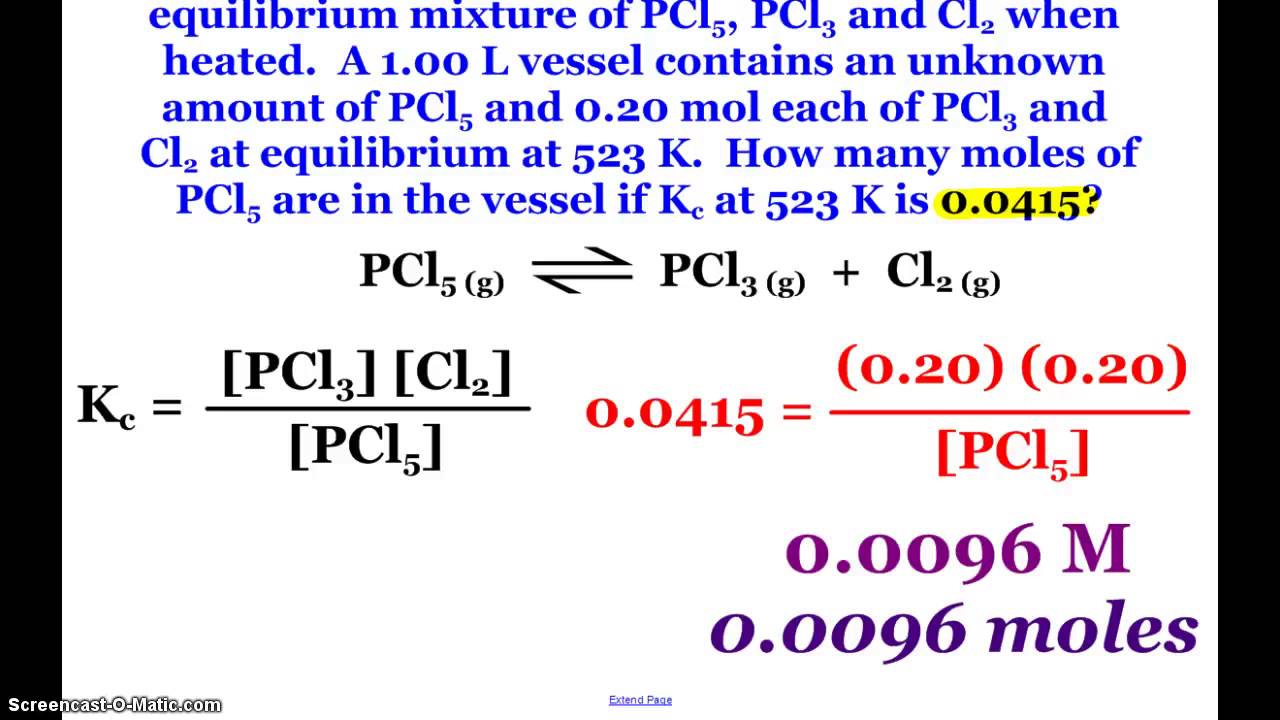
In a container which has a volume Of 11.0 liters and allowed to reach equilibrium at 205 0 C, what would be the concentrations of Br2 (g), C12 (g), and BrCl (g) at equilibrium? What is the numerical value of the equilibrium constant, (g) (g) (g) if the equilibrium constant for the reaction: N2 (g) + 02 (g) is 3.5 x 10-6 2 NO (g). NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔH RXN 0. A sealed rigid vessel contains NH 4 HS(s) in equilibrium with NH 3 (g) and H 2 S(g)as represented by the equation above. Which of the following changes will increase the amount of NH 4 HS(s) in the vessel? College Board, Advanced Placement, AP, AP Central, and the acorn logo are. AP Chemistry Scoring Guidelines from the 2019 Exam Administration Keywords. You may be surprised to learn that there’s a document called Quantitative Skills in the AP Sciences (2018) that is frankly hardly known by any AP Chemistry teachers. Oddly, but again in classic CB website impenetrable style, that link omits the AP chemistry chapter, and one has to do some URL parsing to find it here.
Bozemanscience Resources

Ap Chem Equilibrium Frq Answers
Equilibrium Concept Map
Equilibrium Slideshow
